Seznamy 51+ Atom Neutron
Seznamy 51+ Atom Neutron. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Neutrons are found together with protons in the atomic nucleus. 29/05/2014 · atoms are made of protons, neutrons, and electrons. Neutral atoms have equal numbers of protons and electrons.
Prezentováno Why Study Particle Physics A Brief Introduction
Neutral atoms have equal numbers of protons and electrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). Neutrons are found together with protons in the atomic nucleus. An ion has an unequal number of protons and electrons.Atoms of different elements have different atomic structures because they contain different numbers of …
Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 2) dans le noyau il n'y a que les protons et les neutrons. Atoms of different elements have different atomic structures because they contain different numbers of …

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Neutrons are found together with protons in the atomic nucleus. The number of neutrons in an atom determines its isotope. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 3) l'atome est électriquement neutre donc sa charge est de 0 c. 2) dans le noyau il n'y a que les protons et les neutrons.

Atoms of different elements have different atomic structures because they contain different numbers of … The number of neutrons in an atom determines its isotope. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. An ion has an unequal number of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons.. Neutrons are found together with protons in the atomic nucleus.
L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Neutral atoms have equal numbers of protons and electrons.

The number of neutrons in an atom determines its isotope.. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: A neutral atom has the same number of protons and electrons (charges cancel each other out). Neutrons are found together with protons in the atomic nucleus. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

Neutrons are found together with protons in the atomic nucleus.. An ion has an unequal number of protons and electrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.. A neutral atom has the same number of protons and electrons (charges cancel each other out).

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons... 3) l'atome est électriquement neutre donc sa charge est de 0 c. Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 29/05/2014 · atoms are made of protons, neutrons, and electrons. The number of neutrons in an atom determines its isotope. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Atoms of different elements have different atomic structures because they contain different numbers of … Neutral atoms have equal numbers of protons and electrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

3) l'atome est électriquement neutre donc sa charge est de 0 c. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 2) dans le noyau il n'y a que les protons et les neutrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Atoms of different elements have different atomic structures because they contain different numbers of … Neutral atoms have equal numbers of protons and electrons... Neutrons are found together with protons in the atomic nucleus.

23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 3) l'atome est électriquement neutre donc sa charge est de 0 c. Atoms of different elements have different atomic structures because they contain different numbers of … An ion has an unequal number of protons and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out).. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion... An ion has an unequal number of protons and electrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The number of neutrons in an atom determines its isotope. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Neutrons are found together with protons in the atomic nucleus.. 2) dans le noyau il n'y a que les protons et les neutrons.
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: A neutral atom has the same number of protons and electrons (charges cancel each other out). 3) l'atome est électriquement neutre donc sa charge est de 0 c. An ion has an unequal number of protons and electrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons.

Atoms of different elements have different atomic structures because they contain different numbers of … 3) l'atome est électriquement neutre donc sa charge est de 0 c. The number of neutrons in an atom determines its isotope. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Atoms of different elements have different atomic structures because they contain different numbers of … Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. The number of neutrons in an atom determines its isotope. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons.. Atoms of different elements have different atomic structures because they contain different numbers of …

Atoms of different elements have different atomic structures because they contain different numbers of … Atoms of different elements have different atomic structures because they contain different numbers of … Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral.

The number of neutrons in an atom determines its isotope. The number of neutrons in an atom determines its isotope.. Neutrons are found together with protons in the atomic nucleus.

3) l'atome est électriquement neutre donc sa charge est de 0 c. Atoms of different elements have different atomic structures because they contain different numbers of … 3) l'atome est électriquement neutre donc sa charge est de 0 c. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged... Atoms of different elements have different atomic structures because they contain different numbers of …

L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. An ion has an unequal number of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. A neutral atom has the same number of protons and electrons (charges cancel each other out). The number of neutrons in an atom determines its isotope... Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral.

2) dans le noyau il n'y a que les protons et les neutrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. 2) dans le noyau il n'y a que les protons et les neutrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. A neutral atom has the same number of protons and electrons (charges cancel each other out). 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutral atoms have equal numbers of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 29/05/2014 · atoms are made of protons, neutrons, and electrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

The number of neutrons in an atom determines its isotope... Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Neutral atoms have equal numbers of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. An ion has an unequal number of protons and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral.

3) l'atome est électriquement neutre donc sa charge est de 0 c.. 3) l'atome est électriquement neutre donc sa charge est de 0 c.

Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral... Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Atoms of different elements have different atomic structures because they contain different numbers of … However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0... 2) dans le noyau il n'y a que les protons et les neutrons.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. The number of neutrons in an atom determines its isotope. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 3) l'atome est électriquement neutre donc sa charge est de 0 c. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. The number of neutrons in an atom determines its isotope. 2) dans le noyau il n'y a que les protons et les neutrons. Atoms of different elements have different atomic structures because they contain different numbers of …. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons. The number of neutrons in an atom determines its isotope. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.

Neutrons are found together with protons in the atomic nucleus... A neutral atom has the same number of protons and electrons (charges cancel each other out). 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

An ion has an unequal number of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: An ion has an unequal number of protons and electrons.. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons... However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 29/05/2014 · atoms are made of protons, neutrons, and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. The number of neutrons in an atom determines its isotope. Atoms of different elements have different atomic structures because they contain different numbers of … As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. A neutral atom has the same number of protons and electrons (charges cancel each other out). Atoms of different elements have different atomic structures because they contain different numbers of … Neutrons are found together with protons in the atomic nucleus. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. An ion has an unequal number of protons and electrons. Neutral atoms have equal numbers of protons and electrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. The number of neutrons in an atom determines its isotope. A neutral atom has the same number of protons and electrons (charges cancel each other out).

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Atoms of different elements have different atomic structures because they contain different numbers of … Neutrons are found together with protons in the atomic nucleus. 3) l'atome est électriquement neutre donc sa charge est de 0 c. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 29/05/2014 · atoms are made of protons, neutrons, and electrons. The number of neutrons in an atom determines its isotope.
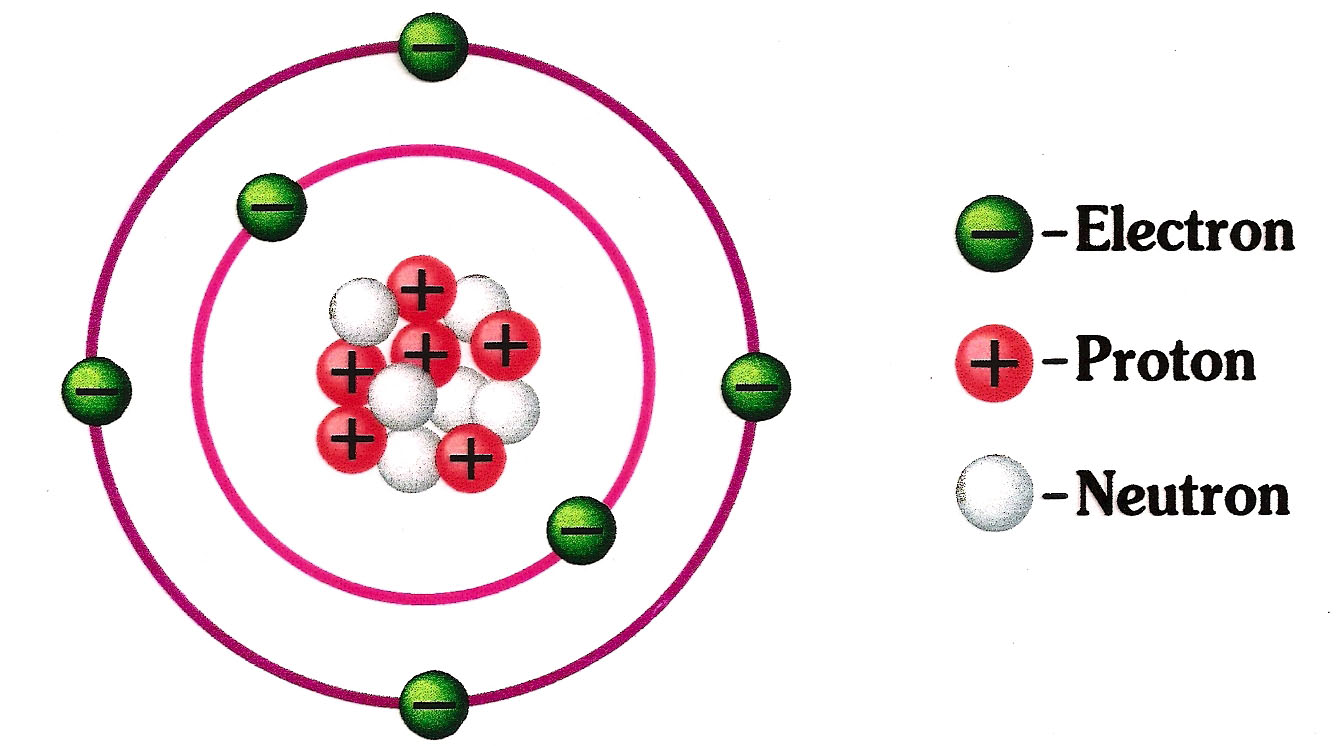
However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Neutrons are found together with protons in the atomic nucleus. 2) dans le noyau il n'y a que les protons et les neutrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. The number of neutrons in an atom determines its isotope.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

The number of neutrons in an atom determines its isotope. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Atoms of different elements have different atomic structures because they contain different numbers of … Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. The number of neutrons in an atom determines its isotope. Neutral atoms have equal numbers of protons and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0... 3) l'atome est électriquement neutre donc sa charge est de 0 c.
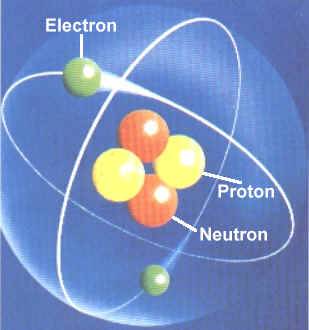
An ion has an unequal number of protons and electrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. An ion has an unequal number of protons and electrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 29/05/2014 · atoms are made of protons, neutrons, and electrons. Neutral atoms have equal numbers of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.

2) dans le noyau il n'y a que les protons et les neutrons. An ion has an unequal number of protons and electrons. Neutral atoms have equal numbers of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutrons are found together with protons in the atomic nucleus. The number of neutrons in an atom determines its isotope. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutral atoms have equal numbers of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. Neutrons are found together with protons in the atomic nucleus. A neutral atom has the same number of protons and electrons (charges cancel each other out). An ion has an unequal number of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. The number of neutrons in an atom determines its isotope. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:. The number of neutrons in an atom determines its isotope.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. . 3) l'atome est électriquement neutre donc sa charge est de 0 c.

29/05/2014 · atoms are made of protons, neutrons, and electrons. Neutrons are found together with protons in the atomic nucleus. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms of different elements have different atomic structures because they contain different numbers of … Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

3) l'atome est électriquement neutre donc sa charge est de 0 c. An ion has an unequal number of protons and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. Neutral atoms have equal numbers of protons and electrons. Atoms of different elements have different atomic structures because they contain different numbers of … The number of neutrons in an atom determines its isotope. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.. Neutrons are found together with protons in the atomic nucleus.

The number of neutrons in an atom determines its isotope.. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 2) dans le noyau il n'y a que les protons et les neutrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutral atoms have equal numbers of protons and electrons.. 2) dans le noyau il n'y a que les protons et les neutrons.

3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.. Atoms of different elements have different atomic structures because they contain different numbers of …

The number of neutrons in an atom determines its isotope. Neutrons are found together with protons in the atomic nucleus. An ion has an unequal number of protons and electrons. Atoms of different elements have different atomic structures because they contain different numbers of … A neutral atom has the same number of protons and electrons (charges cancel each other out).. Atoms of different elements have different atomic structures because they contain different numbers of …

An ion has an unequal number of protons and electrons.. The number of neutrons in an atom determines its isotope.

Atoms of different elements have different atomic structures because they contain different numbers of …. . 3) l'atome est électriquement neutre donc sa charge est de 0 c.

Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. Neutrons are found together with protons in the atomic nucleus. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged... 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). Atoms of different elements have different atomic structures because they contain different numbers of ….. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

Atoms of different elements have different atomic structures because they contain different numbers of … A neutral atom has the same number of protons and electrons (charges cancel each other out). Atoms of different elements have different atomic structures because they contain different numbers of … 3) l'atome est électriquement neutre donc sa charge est de 0 c. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0... Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral.

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. The number of neutrons in an atom determines its isotope. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: A neutral atom has the same number of protons and electrons (charges cancel each other out). 3) l'atome est électriquement neutre donc sa charge est de 0 c. An ion has an unequal number of protons and electrons. Neutrons are found together with protons in the atomic nucleus. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons.
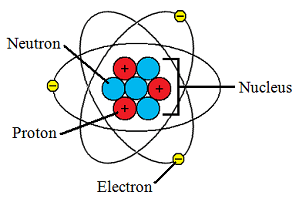
Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 2) dans le noyau il n'y a que les protons et les neutrons. An ion has an unequal number of protons and electrons. Neutrons are found together with protons in the atomic nucleus. Atoms of different elements have different atomic structures because they contain different numbers of … Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. An ion has an unequal number of protons and electrons.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

29/05/2014 · atoms are made of protons, neutrons, and electrons... Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: A neutral atom has the same number of protons and electrons (charges cancel each other out). The number of neutrons in an atom determines its isotope. 2) dans le noyau il n'y a que les protons et les neutrons. Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.

2) dans le noyau il n'y a que les protons et les neutrons. An ion has an unequal number of protons and electrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). 3) l'atome est électriquement neutre donc sa charge est de 0 c. Neutrons are found together with protons in the atomic nucleus. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.

2) dans le noyau il n'y a que les protons et les neutrons. .. 2) dans le noyau il n'y a que les protons et les neutrons.

23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 2) dans le noyau il n'y a que les protons et les neutrons. The number of neutrons in an atom determines its isotope. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms of different elements have different atomic structures because they contain different numbers of … An ion has an unequal number of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons... 29/05/2014 · atoms are made of protons, neutrons, and electrons. Atoms of different elements have different atomic structures because they contain different numbers of …

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

Neutral atoms have equal numbers of protons and electrons.. Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Atoms of different elements have different atomic structures because they contain different numbers of … The number of neutrons in an atom determines its isotope. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Neutral atoms have equal numbers of protons and electrons... However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.
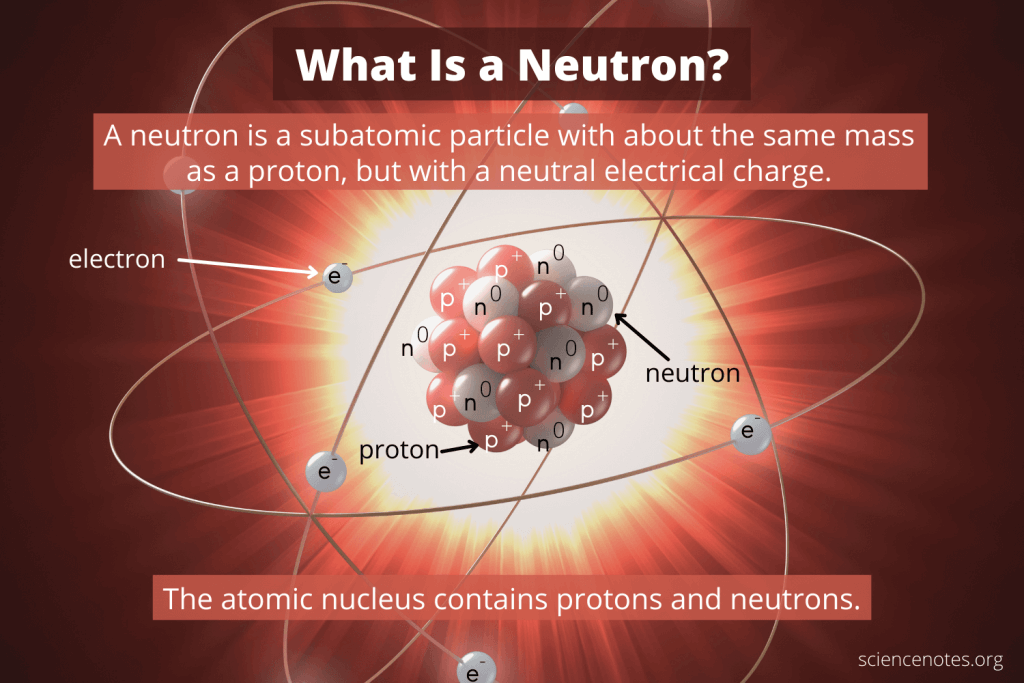
L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.

Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out). L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 3) l'atome est électriquement neutre donc sa charge est de 0 c. The number of neutrons in an atom determines its isotope.

A neutral atom has the same number of protons and electrons (charges cancel each other out). 3) l'atome est électriquement neutre donc sa charge est de 0 c. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 2) dans le noyau il n'y a que les protons et les neutrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral... 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.

3) l'atome est électriquement neutre donc sa charge est de 0 c... 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Neutrons are found together with protons in the atomic nucleus. The number of neutrons in an atom determines its isotope.

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.. An ion has an unequal number of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral... The number of neutrons in an atom determines its isotope.

Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutral atoms have equal numbers of protons and electrons. The number of neutrons in an atom determines its isotope. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. An ion has an unequal number of protons and electrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.. Neutral atoms have equal numbers of protons and electrons.

Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. The number of neutrons in an atom determines its isotope. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Atoms of different elements have different atomic structures because they contain different numbers of … 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 2) dans le noyau il n'y a que les protons et les neutrons. Neutrons are found together with protons in the atomic nucleus.. Neutrons are found together with protons in the atomic nucleus.

Neutral atoms have equal numbers of protons and electrons.. A neutral atom has the same number of protons and electrons (charges cancel each other out). L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. An ion has an unequal number of protons and electrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Neutrons are found together with protons in the atomic nucleus. 2) dans le noyau il n'y a que les protons et les neutrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms of different elements have different atomic structures because they contain different numbers of … 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0... Atoms of different elements have different atomic structures because they contain different numbers of …

2) dans le noyau il n'y a que les protons et les neutrons.. . 3) l'atome est électriquement neutre donc sa charge est de 0 c.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 3) l'atome est électriquement neutre donc sa charge est de 0 c. An ion has an unequal number of protons and electrons. Neutrons are found together with protons in the atomic nucleus. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. Neutral atoms have equal numbers of protons and electrons. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.
29/05/2014 · atoms are made of protons, neutrons, and electrons... A neutral atom has the same number of protons and electrons (charges cancel each other out). 29/05/2014 · atoms are made of protons, neutrons, and electrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutral atoms have equal numbers of protons and electrons. Neutrons are found together with protons in the atomic nucleus.

3) l'atome est électriquement neutre donc sa charge est de 0 c.. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 3) l'atome est électriquement neutre donc sa charge est de 0 c.. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.

Neutrons are found together with protons in the atomic nucleus. 2) dans le noyau il n'y a que les protons et les neutrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. An ion has an unequal number of protons and electrons. A neutral atom has the same number of protons and electrons (charges cancel each other out). 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms of different elements have different atomic structures because they contain different numbers of … However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. The number of neutrons in an atom determines its isotope. Neutral atoms have equal numbers of protons and electrons. The number of neutrons in an atom determines its isotope.

An ion has an unequal number of protons and electrons... A neutral atom has the same number of protons and electrons (charges cancel each other out). 2) dans le noyau il n'y a que les protons et les neutrons. 29/05/2014 · atoms are made of protons, neutrons, and electrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Neutrons are found together with protons in the atomic nucleus. An ion has an unequal number of protons and electrons. Neutral atoms have equal numbers of protons and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Atoms of different elements have different atomic structures because they contain different numbers of …. 2) dans le noyau il n'y a que les protons et les neutrons.
Neutrons are found together with protons in the atomic nucleus. 29/05/2014 · atoms are made of protons, neutrons, and electrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.. Neutrons are found together with protons in the atomic nucleus.
:max_bytes(150000):strip_icc()/Talaj-5c687c6ac9e77c00016759c8.jpg)
Neutral atoms have equal numbers of protons and electrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.. Neutral atoms have equal numbers of protons and electrons... An ion has an unequal number of protons and electrons.

3) l'atome est électriquement neutre donc sa charge est de 0 c... L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. The number of neutrons in an atom determines its isotope. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Neutral atoms have equal numbers of protons and electrons. An ion has an unequal number of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. A neutral atom has the same number of protons and electrons (charges cancel each other out)... 3) l'atome est électriquement neutre donc sa charge est de 0 c.

29/05/2014 · atoms are made of protons, neutrons, and electrons. A neutral atom has the same number of protons and electrons (charges cancel each other out).

23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Neutrons are found together with protons in the atomic nucleus. A neutral atom has the same number of protons and electrons (charges cancel each other out). Neutral atoms have equal numbers of protons and electrons. 2) dans le noyau il n'y a que les protons et les neutrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutrons are found together with protons in the atomic nucleus. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. A neutral atom has the same number of protons and electrons (charges cancel each other out). 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. An ion has an unequal number of protons and electrons.. 2) dans le noyau il n'y a que les protons et les neutrons.

3) l'atome est électriquement neutre donc sa charge est de 0 c.. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 3) l'atome est électriquement neutre donc sa charge est de 0 c.. Atoms of different elements have different atomic structures because they contain different numbers of …

23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The number of neutrons in an atom determines its isotope. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 29/05/2014 · atoms are made of protons, neutrons, and electrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.
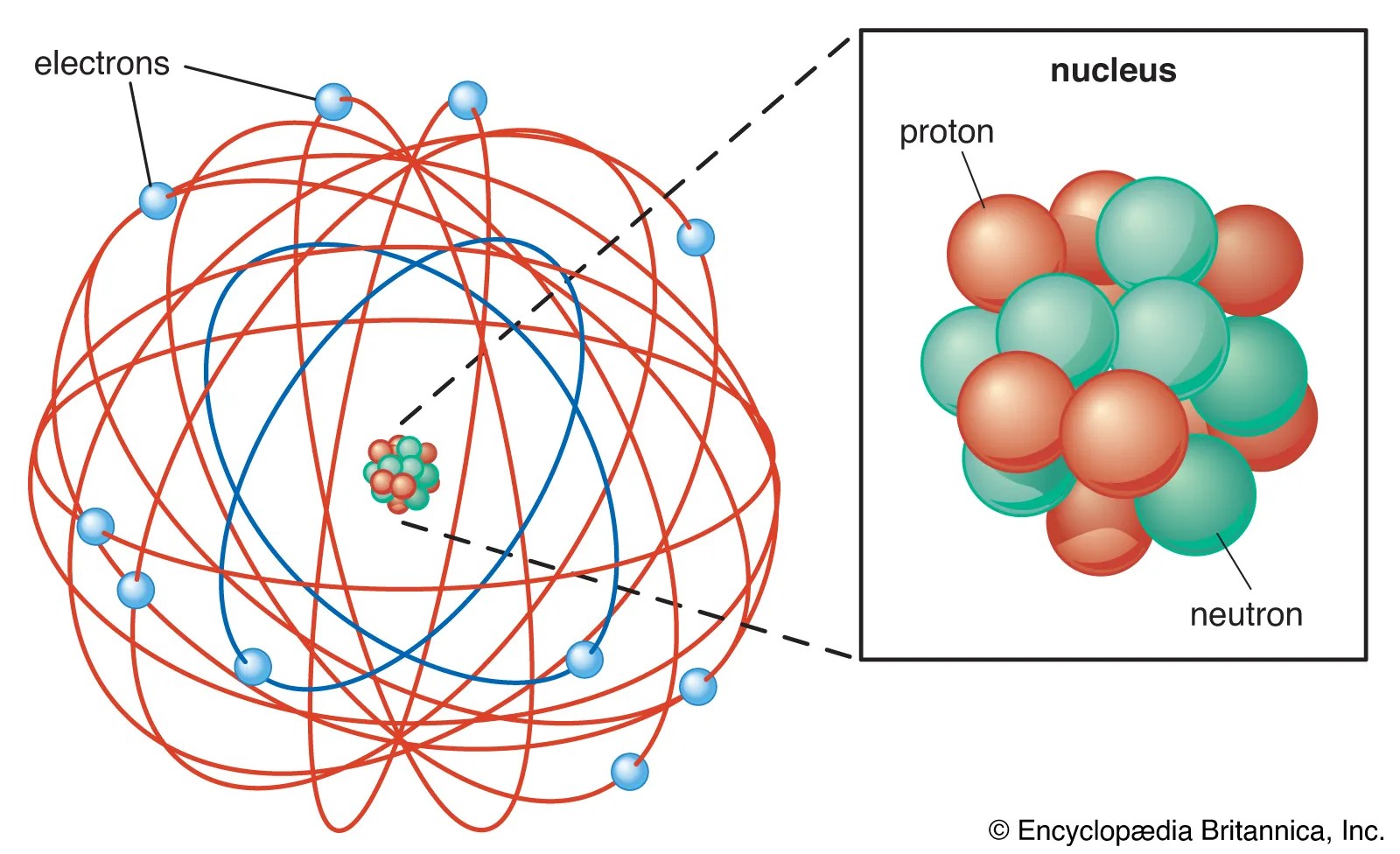
Atoms of different elements have different atomic structures because they contain different numbers of ….. L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Atoms of different elements have different atomic structures because they contain different numbers of … 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The number of neutrons in an atom determines its isotope. A neutral atom has the same number of protons and electrons (charges cancel each other out).. 29/05/2014 · atoms are made of protons, neutrons, and electrons.
A neutral atom has the same number of protons and electrons (charges cancel each other out). 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: An ion has an unequal number of protons and electrons... L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons.

A neutral atom has the same number of protons and electrons (charges cancel each other out).. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutral atoms have equal numbers of protons and electrons.

The number of neutrons in an atom determines its isotope. The number of neutrons in an atom determines its isotope. An ion has an unequal number of protons and electrons. Atoms of different elements have different atomic structures because they contain different numbers of … 2) dans le noyau il n'y a que les protons et les neutrons. 3) l'atome est électriquement neutre donc sa charge est de 0 c... As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

The number of neutrons in an atom determines its isotope. A neutral atom has the same number of protons and electrons (charges cancel each other out). 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0... 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

A neutral atom has the same number of protons and electrons (charges cancel each other out). Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. Neutrons are found together with protons in the atomic nucleus. 2) dans le noyau il n'y a que les protons et les neutrons. Atoms of different elements have different atomic structures because they contain different numbers of … However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Neutrons are found together with protons in the atomic nucleus.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged... Atoms of different elements have different atomic structures because they contain different numbers of … A neutral atom has the same number of protons and electrons (charges cancel each other out). The number of neutrons in an atom determines its isotope. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:.. The number of neutrons in an atom determines its isotope.

Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons.. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutrons are found together with protons in the atomic nucleus. A neutral atom has the same number of protons and electrons (charges cancel each other out). 29/05/2014 · atoms are made of protons, neutrons, and electrons.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion... Neutral atoms have equal numbers of protons and electrons. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: L'atome d'oxygène est donc composé de 8 protons, 7 neutrons et 8 électrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion. 3) l'atome est électriquement neutre donc sa charge est de 0 c. Atoms of different elements have different atomic structures because they contain different numbers of … As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. 29/05/2014 · atoms are made of protons, neutrons, and electrons.. Neutrons are found together with protons in the atomic nucleus.

However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion... The number of neutrons in an atom determines its isotope. 23/09/2019 · all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 3) l'atome est électriquement neutre donc sa charge est de 0 c. Neutral atoms have equal numbers of protons and electrons. Atoms of different elements have different atomic structures because they contain different numbers of … A neutral atom has the same number of protons and electrons (charges cancel each other out). 2) dans le noyau il n'y a que les protons et les neutrons... 3) l'atome est électriquement neutre donc sa charge est de 0 c.

18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Neutrons are found together with protons in the atomic nucleus. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0. Atoms of different elements have different atomic structures because they contain different numbers of … Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. Donc le noyau d'oxygène est composé de 8 protons et 7 neutrons. An ion has an unequal number of protons and electrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 29/05/2014 · atoms are made of protons, neutrons, and electrons. However, atoms may gain or lose electrons in order to increase their stability and the resulting charged entity is called an ion.. 18/12/2008 · the neutron is the particle in the atomic nucleus with a mass = 1 and charge = 0.