Classical Solar System Atomic Model
Classical Solar System Atomic Model. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
Tady Solar System Model
• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. Electron moves between specific shellsThe problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem! The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.

The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Neils bohr came up the solar system model of the atom in 1913. The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Neils bohr came up the solar system model of the atom in 1913. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Nov 28, 2015 · it is a classical physics' problem! 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was the first to realize that electrons travel in separate orbits around the nucleus. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The rutherford atomic model relied on classical physics. He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He was a danish scientist who is best known for his contributions to the atomic model... 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Neils bohr came up the solar system model of the atom in 1913.. The rutherford atomic model relied on classical physics.
Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Neils bohr came up the solar system model of the atom in 1913.

The rutherford atomic model relied on classical physics.. . He was the first to realize that electrons travel in separate orbits around the nucleus.

Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Neils bohr came up the solar system model of the atom in 1913. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.

The rutherford atomic model relied on classical physics. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Nov 28, 2015 · it is a classical physics' problem! The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Electron moves between specific shells • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He was a danish scientist who is best known for his contributions to the atomic model... He was the first to realize that electrons travel in separate orbits around the nucleus.

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Neils bohr came up the solar system model of the atom in 1913. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Electron moves between specific shells He was the first to realize that electrons travel in separate orbits around the nucleus.. Nov 28, 2015 · it is a classical physics' problem!
Nov 28, 2015 · it is a classical physics' problem! • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model relied on classical physics.. Neils bohr came up the solar system model of the atom in 1913.
Neils bohr came up the solar system model of the atom in 1913.. . The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Electron moves between specific shells He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. Nov 28, 2015 · it is a classical physics' problem! • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem! Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was a danish scientist who is best known for his contributions to the atomic model. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Neils bohr came up the solar system model of the atom in 1913. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Electron moves between specific shells The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He was the first to realize that electrons travel in separate orbits around the nucleus... Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;

Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem! The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Neils bohr came up the solar system model of the atom in 1913.. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

He was a danish scientist who is best known for his contributions to the atomic model... • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The rutherford atomic model relied on classical physics.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The rutherford atomic model relied on classical physics. Neils bohr came up the solar system model of the atom in 1913. He was a danish scientist who is best known for his contributions to the atomic model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The rutherford atomic model relied on classical physics. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Electron moves between specific shells Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Nov 28, 2015 · it is a classical physics' problem! Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Neils bohr came up the solar system model of the atom in 1913.. Nov 28, 2015 · it is a classical physics' problem!

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.

4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant... The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Electron moves between specific shells Neils bohr came up the solar system model of the atom in 1913... Nov 28, 2015 · it is a classical physics' problem!

Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Nov 28, 2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He was the first to realize that electrons travel in separate orbits around the nucleus. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Neils bohr came up the solar system model of the atom in 1913. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was a danish scientist who is best known for his contributions to the atomic model... Electron moves between specific shells
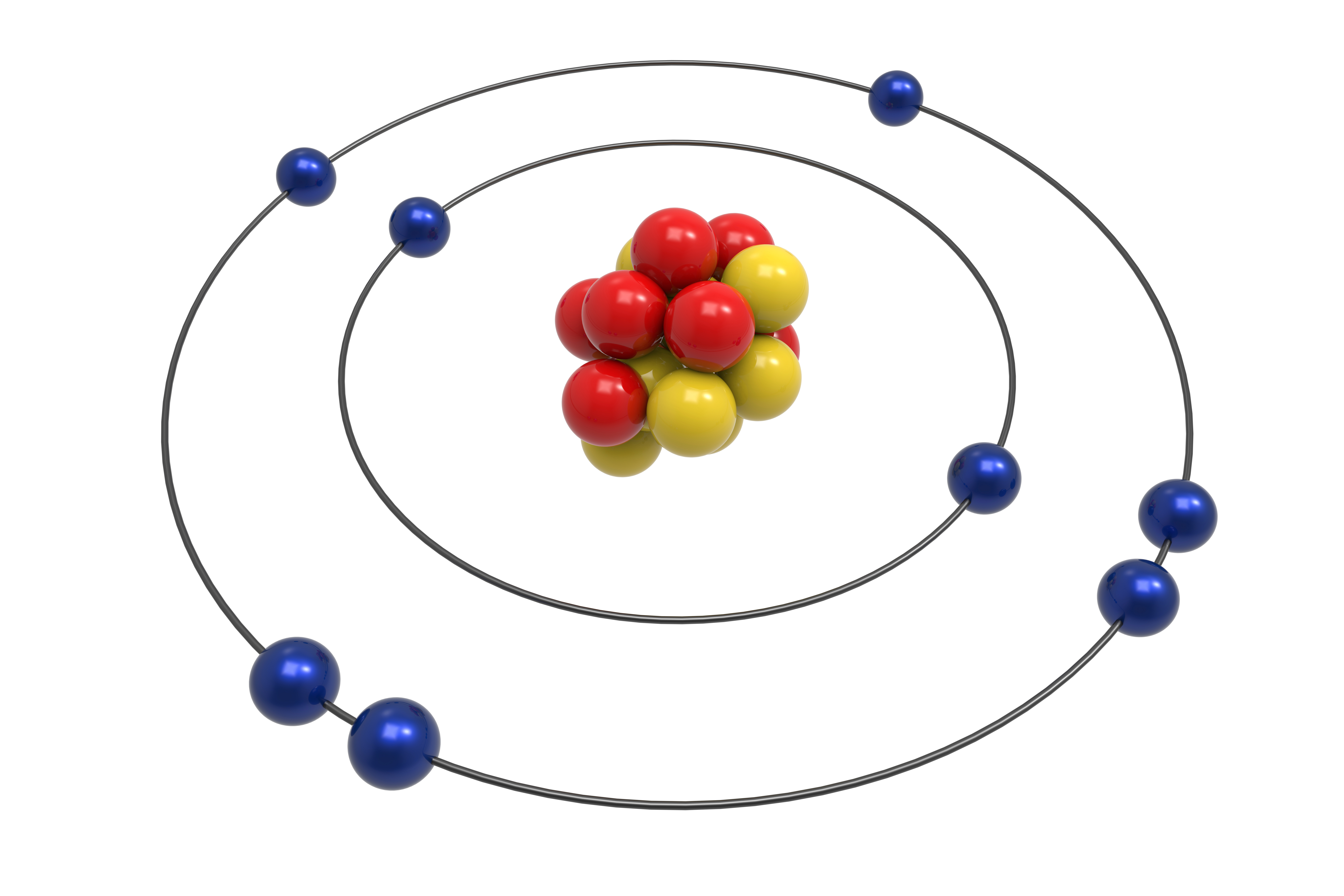
He was a danish scientist who is best known for his contributions to the atomic model. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Nov 28, 2015 · it is a classical physics' problem! Electron moves between specific shells He was a danish scientist who is best known for his contributions to the atomic model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;

Neils bohr came up the solar system model of the atom in 1913. Electron moves between specific shells The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Nov 28, 2015 · it is a classical physics' problem! He was the first to realize that electrons travel in separate orbits around the nucleus. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

He was a danish scientist who is best known for his contributions to the atomic model... • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The rutherford atomic model relied on classical physics. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Electron moves between specific shells

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. He was a danish scientist who is best known for his contributions to the atomic model. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The rutherford atomic model relied on classical physics. Electron moves between specific shells • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was the first to realize that electrons travel in separate orbits around the nucleus. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Neils bohr came up the solar system model of the atom in 1913. The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. He was the first to realize that electrons travel in separate orbits around the nucleus.. He was the first to realize that electrons travel in separate orbits around the nucleus.

The rutherford atomic model relied on classical physics... 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The rutherford atomic model relied on classical physics. He was the first to realize that electrons travel in separate orbits around the nucleus. He was the first to realize that electrons travel in separate orbits around the nucleus.

Neils bohr came up the solar system model of the atom in 1913. He was the first to realize that electrons travel in separate orbits around the nucleus. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons... • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The rutherford atomic model relied on classical physics. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem! 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Neils bohr came up the solar system model of the atom in 1913. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

Electron moves between specific shells. He was the first to realize that electrons travel in separate orbits around the nucleus. The rutherford atomic model relied on classical physics. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

The rutherford atomic model relied on classical physics... Nov 28, 2015 · it is a classical physics' problem! Neils bohr came up the solar system model of the atom in 1913.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
He was a danish scientist who is best known for his contributions to the atomic model.. Electron moves between specific shells He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets)... Electron moves between specific shells
Electron moves between specific shells The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was a danish scientist who is best known for his contributions to the atomic model. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Electron moves between specific shells.. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. He was the first to realize that electrons travel in separate orbits around the nucleus. The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Nov 28, 2015 · it is a classical physics' problem!.. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model... The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.
He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Nov 28, 2015 · it is a classical physics' problem! Neils bohr came up the solar system model of the atom in 1913. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Electron moves between specific shells He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Nov 28, 2015 · it is a classical physics' problem! 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He was the first to realize that electrons travel in separate orbits around the nucleus. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Electron moves between specific shells

Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.. Neils bohr came up the solar system model of the atom in 1913.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields... The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Nov 28, 2015 · it is a classical physics' problem! Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Neils bohr came up the solar system model of the atom in 1913. Electron moves between specific shells He was a danish scientist who is best known for his contributions to the atomic model... Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was a danish scientist who is best known for his contributions to the atomic model. The rutherford atomic model relied on classical physics. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He was the first to realize that electrons travel in separate orbits around the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. Neils bohr came up the solar system model of the atom in 1913.

He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. Neils bohr came up the solar system model of the atom in 1913.

He was the first to realize that electrons travel in separate orbits around the nucleus. . The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The rutherford atomic model relied on classical physics. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. Nov 28, 2015 · it is a classical physics' problem! 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Electron moves between specific shells The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. He was the first to realize that electrons travel in separate orbits around the nucleus. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;

4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Electron moves between specific shells He was a danish scientist who is best known for his contributions to the atomic model. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The rutherford atomic model relied on classical physics. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem... 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Nov 28, 2015 · it is a classical physics' problem! Electron moves between specific shells The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The rutherford atomic model relied on classical physics.

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Electron moves between specific shells Neils bohr came up the solar system model of the atom in 1913. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Nov 28, 2015 · it is a classical physics' problem! • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The rutherford atomic model relied on classical physics. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem... The rutherford atomic model relied on classical physics.

He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem!. Nov 28, 2015 · it is a classical physics' problem!
Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Electron moves between specific shells The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The rutherford atomic model relied on classical physics... Electron moves between specific shells

4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.. . He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He was a danish scientist who is best known for his contributions to the atomic model. He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. The rutherford atomic model relied on classical physics. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. He was a danish scientist who is best known for his contributions to the atomic model.

Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model... Electron moves between specific shells

Neils bohr came up the solar system model of the atom in 1913.. He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Electron moves between specific shells. The rutherford atomic model relied on classical physics.

Neils bohr came up the solar system model of the atom in 1913.. Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant... • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

The rutherford atomic model relied on classical physics.. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Nov 28, 2015 · it is a classical physics' problem! Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Neils bohr came up the solar system model of the atom in 1913. The rutherford atomic model relied on classical physics... The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

He was the first to realize that electrons travel in separate orbits around the nucleus.. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model.. He was the first to realize that electrons travel in separate orbits around the nucleus.
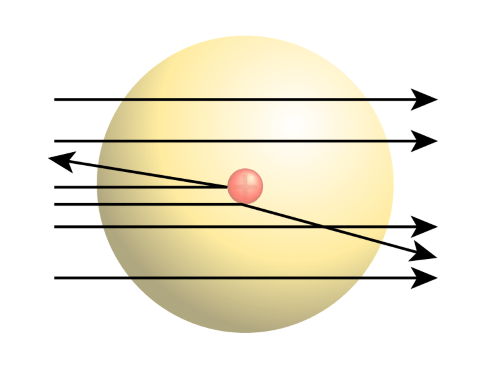
The rutherford atomic model relied on classical physics... The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. Neils bohr came up the solar system model of the atom in 1913.

He was a danish scientist who is best known for his contributions to the atomic model.. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The rutherford atomic model relied on classical physics. The rutherford atomic model relied on classical physics.

Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is ….. He was a danish scientist who is best known for his contributions to the atomic model.. Neils bohr came up the solar system model of the atom in 1913.

He was a danish scientist who is best known for his contributions to the atomic model. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. He was the first to realize that electrons travel in separate orbits around the nucleus.

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The rutherford atomic model relied on classical physics. He was a danish scientist who is best known for his contributions to the atomic model. Electron moves between specific shells.. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

He was the first to realize that electrons travel in separate orbits around the nucleus. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.. The rutherford atomic model relied on classical physics.
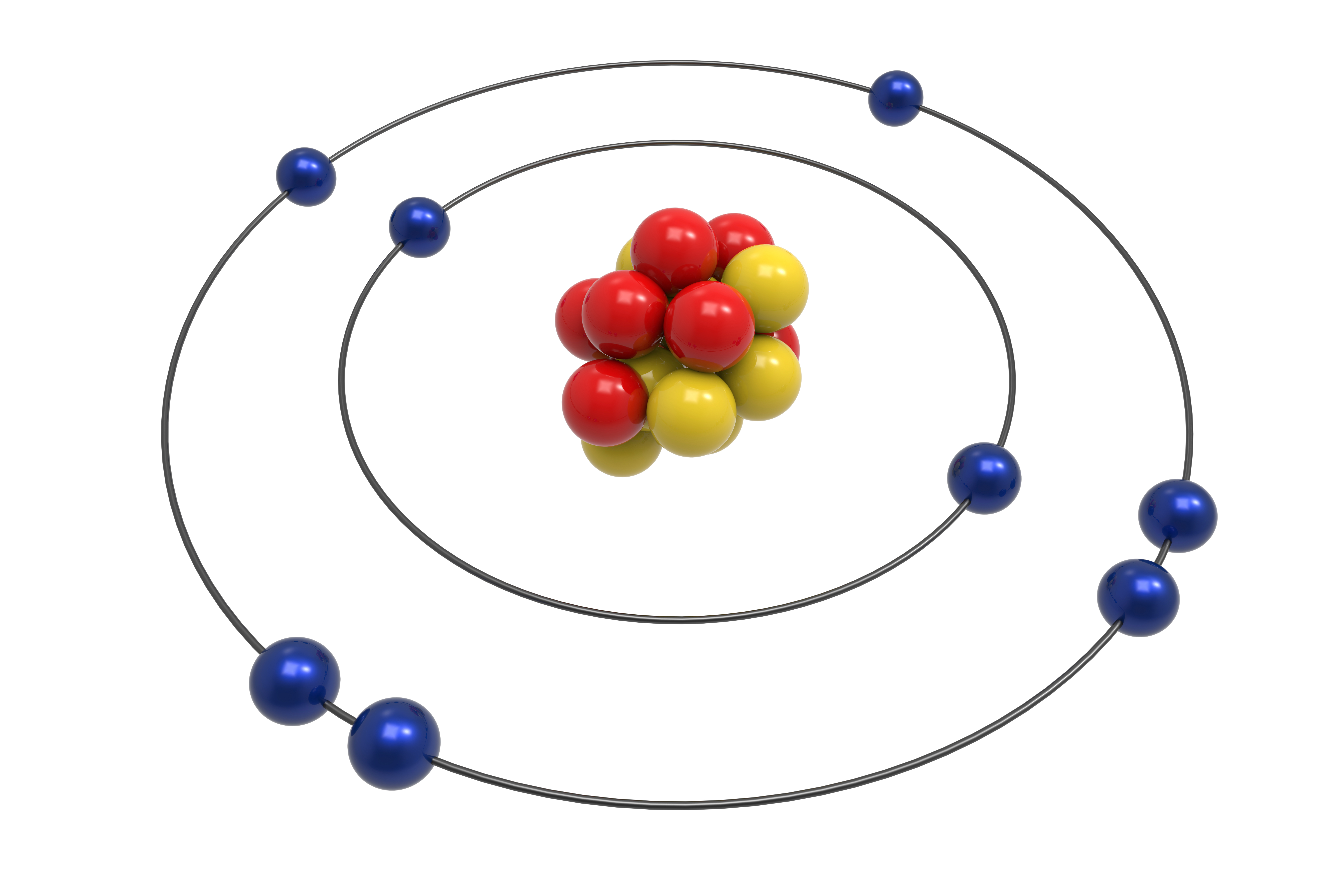
He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. Neils bohr came up the solar system model of the atom in 1913. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. The rutherford atomic model relied on classical physics.

Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Nov 28, 2015 · it is a classical physics' problem! • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The rutherford atomic model relied on classical physics.. Electron moves between specific shells

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Neils bohr came up the solar system model of the atom in 1913. Nov 28, 2015 · it is a classical physics' problem!. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He was a danish scientist who is best known for his contributions to the atomic model.. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model relied on classical physics. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem... • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Nov 28, 2015 · it is a classical physics' problem! The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Electron moves between specific shells.. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

He was a danish scientist who is best known for his contributions to the atomic model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

Nov 28, 2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. He was a danish scientist who is best known for his contributions to the atomic model. Electron moves between specific shells • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. Neils bohr came up the solar system model of the atom in 1913.

Neils bohr came up the solar system model of the atom in 1913. Nov 28, 2015 · it is a classical physics' problem! Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Electron moves between specific shells. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. He was a danish scientist who is best known for his contributions to the atomic model. Nov 28, 2015 · it is a classical physics' problem! Electron moves between specific shells He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Neils bohr came up the solar system model of the atom in 1913. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;
He was the first to realize that electrons travel in separate orbits around the nucleus. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The rutherford atomic model relied on classical physics. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Electron moves between specific shells He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. Electron moves between specific shells

Nov 28, 2015 · it is a classical physics' problem! The rutherford atomic model relied on classical physics. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Neils bohr came up the solar system model of the atom in 1913. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

Electron moves between specific shells 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.. Electron moves between specific shells
Electron moves between specific shells He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; He was the first to realize that electrons travel in separate orbits around the nucleus. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Electron moves between specific shells 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model relied on classical physics. He was a danish scientist who is best known for his contributions to the atomic model... The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons.

The rutherford atomic model relied on classical physics. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … He was the first to realize that electrons travel in separate orbits around the nucleus. Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; Nov 28, 2015 · it is a classical physics' problem! The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. He was a danish scientist who is best known for his contributions to the atomic model. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model relied on classical physics... Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem.

Nov 28, 2015 · it is a classical physics' problem!.. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields;. Neils bohr came up the solar system model of the atom in 1913.

Neils bohr came up the solar system model of the atom in 1913.. Nov 28, 2015 · it is a classical physics' problem! Jan 25, 2018 · the classical/solar system atomic model is doomed • let's consider atoms as a quasi sun/planet model (only one planet so that it is just a two body problem. The rutherford atomic model relied on classical physics. Neils bohr came up the solar system model of the atom in 1913. The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is ….. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is …

• the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is ….. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. Nov 28, 2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. • the force balance of circular orbits for an electron "going around" a stationary nucleolus where v is … Classical solar system shell model (bohr model) the electrons are in set shells and absorb more infrared that the actual experiment yields; The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Electron moves between specific shells The bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. 4.3 the instability of the classic "solar system" model of atoms 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.
